Logohu në mjekët.al
BENZODIAZEPINAT – BZD
The first benzodiazepine, chlordiazepoxide (librium), was synthesized in 1955 by Leo Sternbach, while he was working for Hoffmann-La Roche on the production of tranquilizers. The pharmacological properties of the compounds initially prepared were disappointing, and Sternbach abandoned the project. Two years later, in April of 1957, his colleague, Earl Reeder, noticed a "fine crystalline" compound left over from the discontinued project while cleaning the laboratory. This compound, later called chlordiazepoxide, had not been tested in 1955 due to Sternbach's focus on other issues. The compound showed very strong sedative, anticonvulsive, and muscle relaxant effects. These impressive clinical results led to its rapid introduction to the market worldwide, in 1960, under the brand name Librium.
After chlordiazepoxide, diazepam was marketed by Hoffmann-La Roche under the brand name Valium, in 1963, and for some time, both drugs were the most commercially successful.
The introduction of benzodiazepines led to a decline in medical prescriptions for barbiturates, and, since 1970, benzodiazepines have largely replaced the older drugs for sedative and hypnotic purposes.
The new group of drugs was initially met with optimism by the medical community, but concerns gradually arose. In particular, the risk of dependency became apparent in the 1980s.
Benzodiazepines have a unique history in that they were responsible for the largest lawsuit ever filed by society against drug manufacturers in the United Kingdom, involving 14,000 patients and 1,800 law firms, which assumed that the manufacturers knew the possibility of dependence but deliberately kept this information hidden from doctors. At the same time, 117 general practitioners and 50 health authorities were sued by patients to compensate for damages from the harmful effects of dependence and withdrawal syndrome. This led to the situation where some doctors began to seek a signed consent form from their patients and recommended that all patients be adequately warned about the risks of dependence and withdrawal before starting treatment with benzodiazepines.
No decision was reached in the legal case against the drug manufacturers. Legal aid was withdrawn, and there were accusations that psychiatric consultants and expert witnesses had a conflict of interest. This legal case led to changes in British law, making it more difficult for society to file lawsuits.
In 2010, documents from a meeting of experts at the Medical Research Council (MRC) in the United Kingdom, previously classified, revealed that the MRC was aware, 30 years ago, from research suggesting that benzodiazepines could cause brain damage in some people, similar to that which occurs from alcohol use, and had failed to continue with larger clinical trials.
The MRC dismissed research proposals, in the 1980s, from Professor Lader and proposals from Professor Ashton, in 1995, to study whether benzodiazepines have permanent effects on the brain.
Although antidepressants with anxiolytic properties have been introduced and there is an increased awareness of the side effects of benzodiazepines, medical prescriptions for the short-term relief of anxiety have not significantly decreased. Now, for the treatment of insomnia, benzodiazepines are used less.
GENERAL INFORMATION
Benzodiazepines (BZD) are widely used in medicine for the treatment of anxiety (anxiolytics) and insomnia (sedatives/hypnotics), as well as for other pathological conditions such as panic attacks and disorders associated with them.
They are synthetic substances, normally produced by the pharmaceutical industry and presented in the form of tablets, pills, and, occasionally, in injectable solution form.
They work by enhancing the activity of the central nervous system (CNS).
Benzodiazepines are under international control.
Diazepam is one of the best-known benzodiazepines (Valium®).
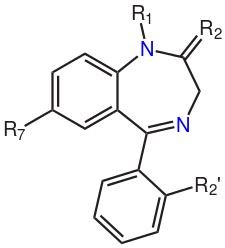
The core structure of benzodiazepines. The "R" notations indicate the common positions of side chains, which give different benzodiazepines their unique properties.
The rate at which different benzodiazepines are metabolized into active forms and their half-life periods vary.
There are products:
- With fast action (e.g., Midazolam) have a half-life period of less than 24 hours.
- With moderate action (e.g., Nitrazepam) have a half-life period of more than 24 hours.
- With long action (e.g., Diazepam) have a half-life period of more than 48 hours.
The half-life period varies according to the individual and, in elderly subjects, these substances tend to be eliminated more slowly. For this reason, in these subjects, there is a higher risk of the appearance of undesirable effects such as drowsiness, ataxia (staggering), mental confusion, judgment alteration, and anterograde amnesia.
Benzodiazepines are generally swallowed in tablet form, but they can be injected for medical or non-medical purposes, and sometimes, non-legal use by nasal route («sniffed») is reported.
33 benzodiazepines were registered in 1984 in Schedule IV of the UN convention of 1971 on psychotropic substances.
List of benzodiazepines under international control
| Name | Duration of action | Main commercial name | CAS No. | |
|---|---|---|---|---|
| Sedatives/hypnotics | ||||
| Brotizolam | Short | Lendormin® | 57801-81-7 | |
| Estazolam | Medium | Pro-Som® | 29975-16-4 | |
| Flunitrazepam | Short/Medium | Rohypnol® | 1622-62-4 | |
| Flurazepam | Long | Dalmane® | 17617-23-1 | |
| Haloxazolam | Long | Somelin® | 59128-97-1 | |
| Loprazolam | Medium | Dormonoct® | 61197-73-7 | |
| Lormetazepam | Short | Noctamid® | 848-75-9 | |
| Midazolam | Short | Versed® | 59467-70-8 | |
| Designation | Duration of action | Main commercial name | CAS No. | Nimetazemap | Long | Erinin® | 2011-67-8 |
|---|---|---|---|
| Nitrazepam | Medium | Mogadon® | 146-22-5 |
| Temazepam | Short | Normison® | 846-50-4 |
| Triazolam | Short | Halcion® | 28911-01-5 |
| Anxiolytics | |||
| Alprazolam | Short | Xanax® | 20981-97-7 |
| Bromazepam | Long | Lexotan® | 1812-30-2 |
| Camazepam | Albego® | 36104-80-2 | |
| Chlordiazepoxide | Long | Librium® | 438-41-5 |
| Clobazam | Long | Frisium® | 22316-47-8 |
| Clonazepam | Medium | Rivotril® | 1622-61-3 |
| Clorazepate | Long | Tranxene® | 57109-90-7 |
| Clotiazepam | Short | Trecalmo® | 33671-46-7 |
| Cloxazolam | Long | Sepazon® | 24166-13-0 |
| Delorazepam | Long | En® | 2894-67-9 |
| Diazepam | Long | Valium® | 439-14-5 |
| Ethyl oflazepate | Long | Meilax® | 29177-84-2 |
| Fludiazepam | Short | Erispan® | 3900-31-00 |
| Halazepam | Long | Pacinone® | 23092-17-3 |
| Ketazolam | Long | Anseren® | 27223-49-1 |
| Lorazepam | Short/Medium | Ativan® | 846-49-1 |
| Medazepam | Long | Nobrium® | 2898-12-6 |
| Nordazepam | Long | Stilny® | 1088-11-5 |
| Oxazepam | Short | Serax® | 604-75-1 |
| Oxazolam | Long | Tranquit® | 27167-30-2 |
| Pinazepam | Long | Domar® | 52463-83-9 |
| Prazepam | Long | Centrax® | 2955-38-6 |
| Tetrazepam | Short | Clinoxan® | 10379-14-3 |
Midazolam (1990) and brotizolam (1995) were added to the list later. In 1995, flunitrazepam was transferred from List IV to List III when the International Narcotics Control Board (INCB) declared that we were dealing with one of the most frequently used benzodiazepines as a narcotic and destined to enter the illicit market.
Fenazepam, which is used in medical practice in some countries outside the EU, is not listed in List IV of the 1971 Convention on Psychotropic Substances.
The INCB emphasized that in 2006, the total legal production of benzodiazepines in the world was at least 180 tons, of which 56 tons were diazepam.
During the period 1997 - 2006, the main producing countries were Italy (32%), India (19%), China (11%), and Germany (10%).
The INCB statistics for 2008 show that Europe reached the first position in terms of average consumption of sedative-hypnotics and anxiolytics, expressed for statistical purposes in defined daily doses (DDD). In 2007, the global consumption of anxiolytics was 27 billion DDD, in 2008, consumption was limited to 27.5 billion DDD.
The results of the ESPAD survey (European School survey Project on Alcohol and other Drugs), in 2007, showed that, on average, 8% of students aged 15-16 from 35 European countries had already tried tranquilizers (sedatives) or sedatives without a doctor's prescription, and this was most frequent in Poland (18%), followed by Lithuania (16%), France (15%), and Italy (10%). Distribution by sex highlights that girls are more numerous in declaring that they have used tranquilizers or sedatives without a prescription.
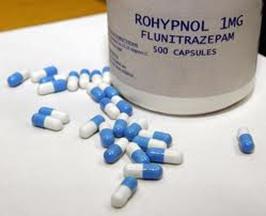
Rohypnol (Flunitrazepam) – 1 mg capsule
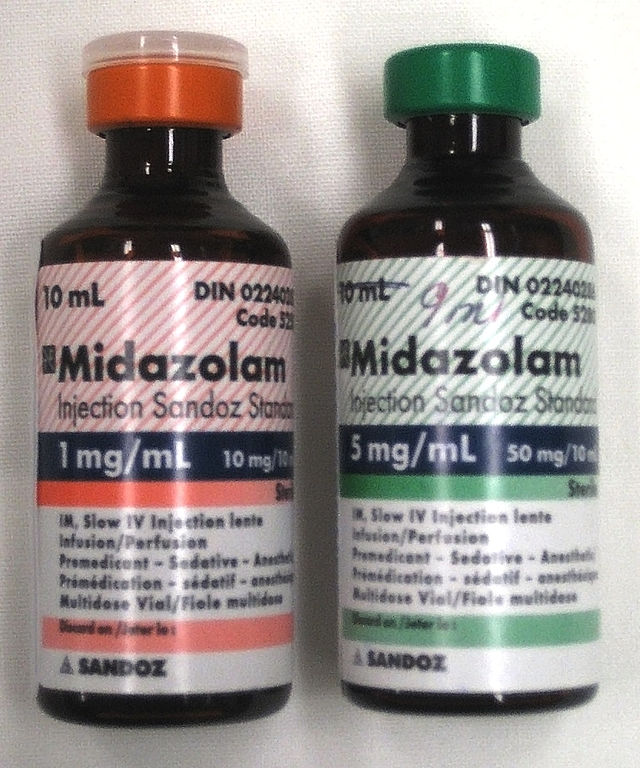
Midazolam injections 1 mg/ml and 5 mg/ml
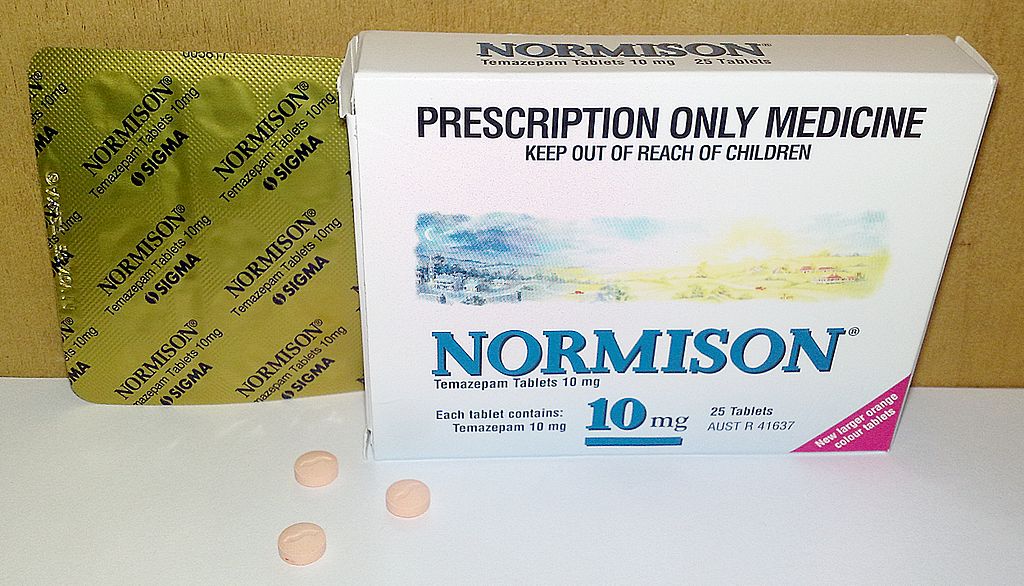
Normison (temazepam) - 10 mg tablet

Diazepam – 2 mg and 5 mg tablets, which are commonly used in benzodiazepine withdrawal treatment.

Xanax (alprazolam) – three-part 2 mg tablets
SHORT-TERM EFFECTS
There is a disinhibition that eventually leads to hostile and aggressive behavior and this is more frequent when benzodiazepines are taken along with alcohol. The combined consumption with alcohol and benzodiazepines also increases the risk of a fatal overdose, since we are dealing with two cases of CNS depressants.
A similar fatal interaction can be produced when opiates are taken with benzodiazepines. A considerable number of problematic drug users swallow, snort, or inject high doses of benzodiazepines, aiming to increase the euphoric effects of opiates, or to reduce the undesirable effects of psychostimulants.
There is a risk of developing an increased dependence on benzodiazepines. Benzodiazepines used for medical purposes should be reserved for the short-term treatment of anxiety, or insomnia, when these are severe and debilitating. A tolerance and a dependence can only appear after several weeks of use. The signs and symptoms of a withdrawal state (weaning, abstention) can be classified as major or minor, similarly to those of the alcoholic syndrome. According to this classification, minor symptoms are restlessness, insomnia, and anxiety, while major symptoms are perceptual disturbances, psychosis, hyperpyrexia, and severe convulsions (spasms).